Mo Maniruzzaman
Chair and Professor of Pharmaceutics and Drug Delivery and Research Professor in the Research Institute of Pharmaceutical Sciences
Research Interests
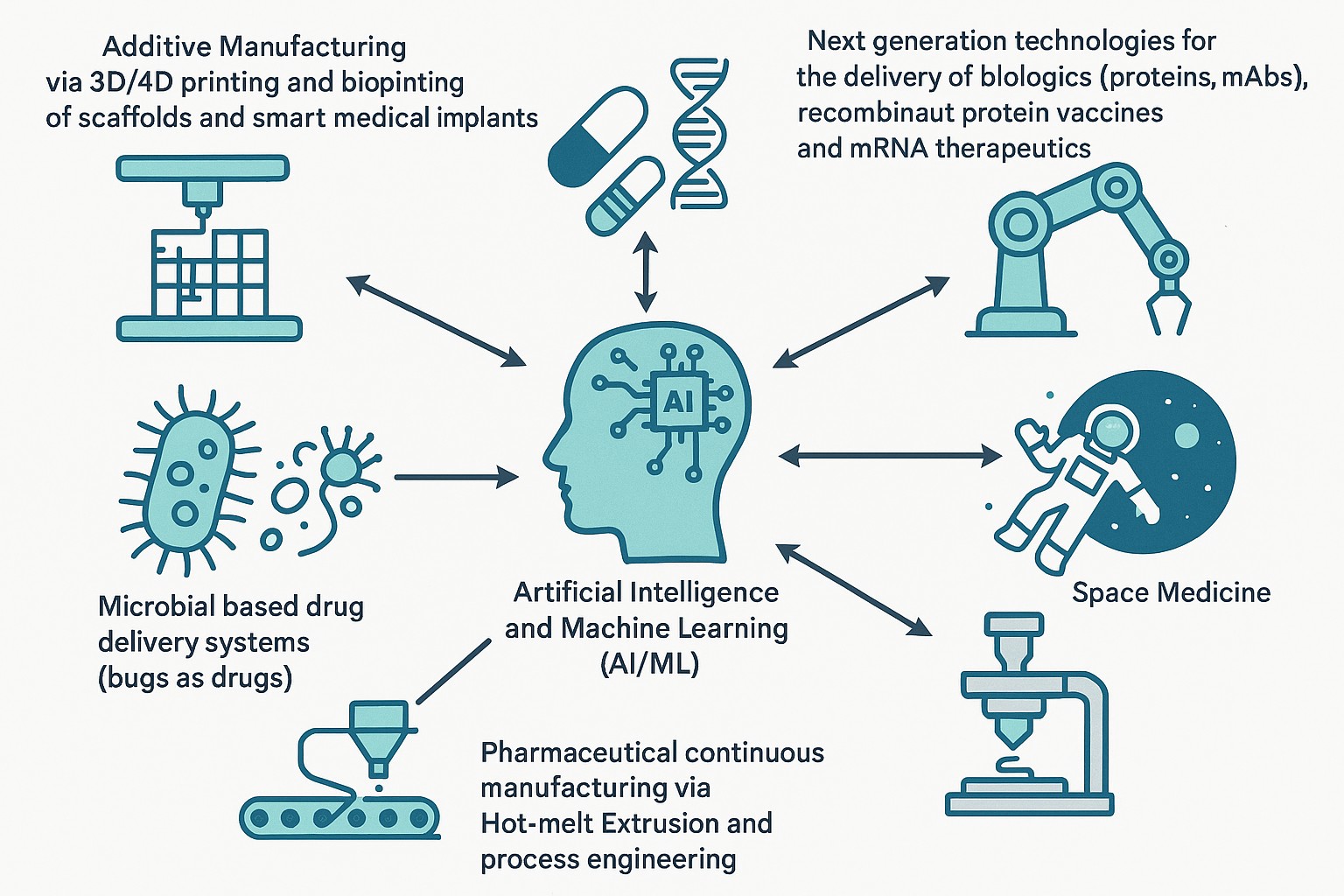
Work in Dr. Maniruzzaman’s lab focuses on pharmaceutical process engineering, continuous manufacturing and 3D printing of medicines including biologics and mRNA therapeutics, AI/ML, robotics and space medicine. Additional projects focus on 3D bioprinting of scaffolds and smart medical implants as well as ultra-portable drug delivery devices.
Biography
Dr. Mo Maniruzzaman is Chair and Professor of Pharmaceutics & Drug Delivery, Research Professor in the Research Institute of Pharmaceutical Sciences (RIPS) at the University of Mississippi School of Pharmacy and leads the PharmE3D Lab. Previously, he was a faculty member at UT Austin College of Pharmacy and the University of Sussex, UK. He earned his PhD at 23 from the University of Greenwich, UK with two accompanying awards including the Vice Chancellor’s Award in 2013 for outstanding performance in his doctoral research and, at 34, became the youngest Chair and Professor at an R1 university and a School of Pharmacy in the U.S. His research focuses on 3D printing/4D printing, pharmaceutical engineering, advanced drug delivery, AI/ML, and space medicine, reflected in 100+ publications, >30 patents and patent applications, several proprietary technologies licensed to industry. He has received >25 prestigious awards/accolades, secured federal and industry grants (~$9.5 million since 2019 and >$22 million pending), and serves as an editor for AAPS Open.
Education
B.S. Pharmaceutical Science, University of Greenwich (2009)
Ph.D. Pharmaceutical Science, University of Greenwich (2013)
